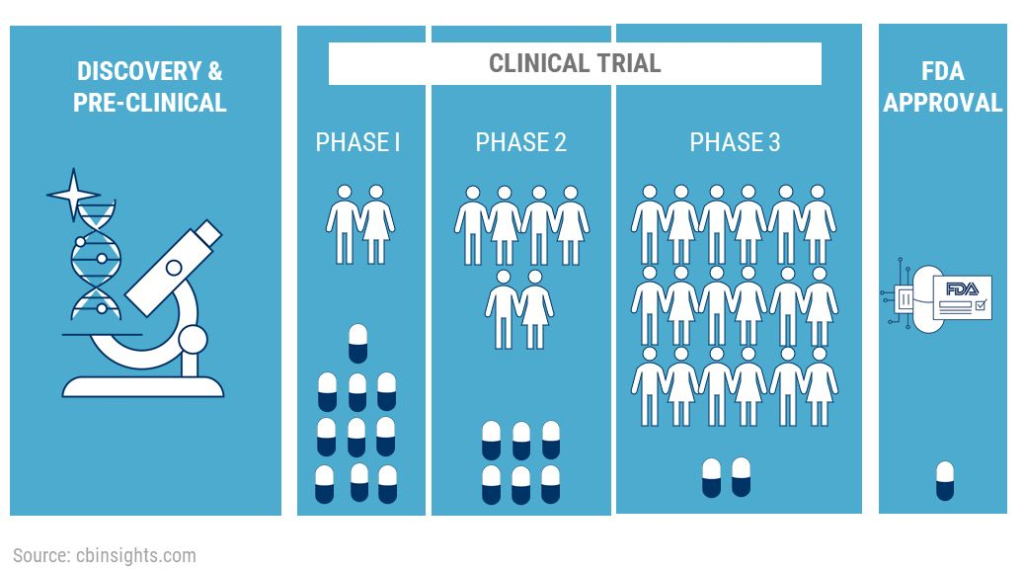
New clinical trial upcoming using cyclodextrin as active
Cyclo Therapeutics, Inc. sponsors Phase II trial for early Alzheimer’s disease. The study will enroll approximately 90 male and female patients aged 50 to 80 years who will be given a minimum active dose of 500 mg/kg of Trappsol Cyclo (equivalent to 18,500 mg/m2) as an intravenous (IV) infusion once every 28 days for 6 months.
We wish the best of success to our colleagues at Cyclo Therapeutics and hope this therapy will prove useful for the benefit of these patients.
Having programs ourselves in developing NCEs for neurodegenerative diseases, we find this study particularly essential.
Read more about the clinical trial here