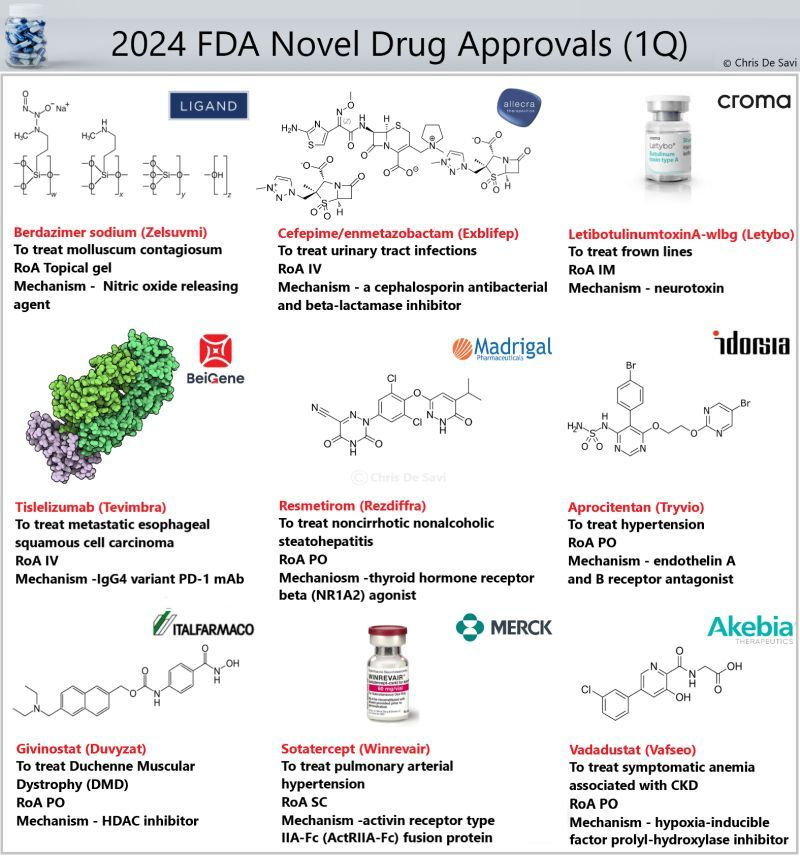
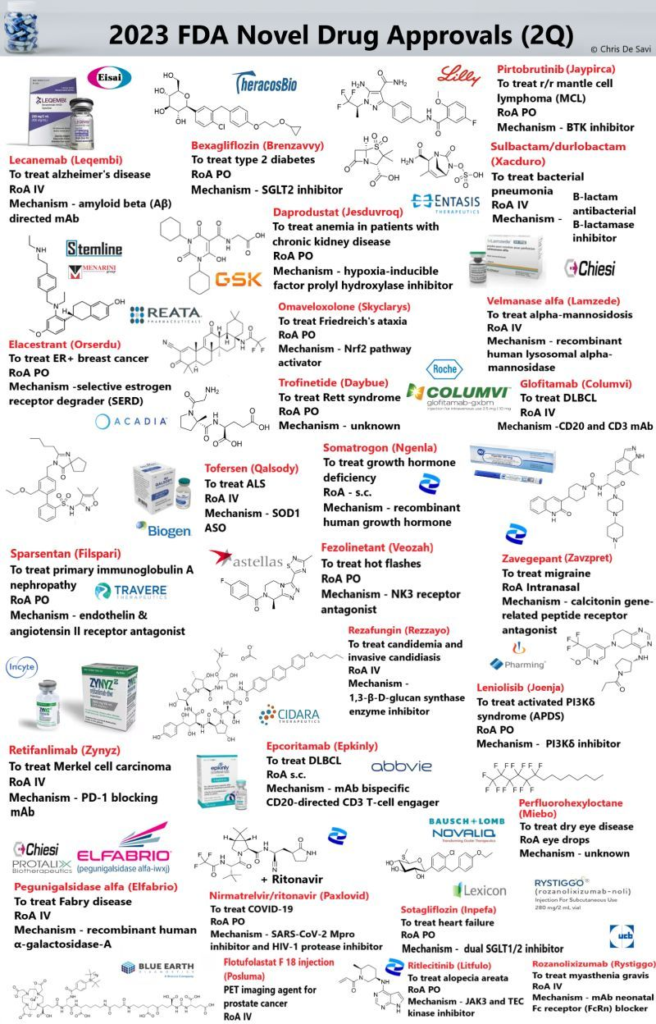
26 new drugs approved by the FDA’s Center for Drug Evaluation and Research (CDER) for 1H! Lots of exciting new mechanisms and modalities.
Highlights from the 2Q include –
💉 Tofersen (Qalsody), an antisense oligonucleotide indicated for the treatment of adult amyotrophic lateral sclerosis caused by SOD1 mutation.
💉 Pegunigalsidase alfa (Elfabrio), a recombinant form of human α-galactosidase-A indicated for long-term enzyme replacement therapy in patients with Fabry disease.
💊 Fezolinetant (Veozah), a selective neurokinin-3 (NK3) receptor antagonist used to treat moderate to severe vasomotor symptoms due to menopause.
👁 Perfluorohexyloctane (Miebo) is a drug used to treat dry eye disease. Check out structure! Mechanism unknown.
💉Epcoritamab (Epkinly), a bispecific CD20-directed CD3 T-cell engager used to treat relapsed or refractory diffuse large B-cell lymphoma (DLBCL) in adults.
💊 Sulbactam/durlobactam (Xacduro), a co-packaged medication used for the treatment of bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus complex. It contains sulbactam, a beta-lactam antibacterial and beta-lactamase inhibitor; and durlobactam, a beta-lactamase inhibitor.
💊 Nirmatrelvir/ritonavir (Paxlovid), a co-packaged medication used as a treatment for COVID‑19. It contains the antiviral medications nirmatrelvir and ritonavir. Nirmatrelvir is a SARS-CoV-2 main protease inhibitor while ritonavir is a HIV-1 protease inhibitor and strong CYP3A4 inhibitor.
💉Flotufolastat F-18 (Posluma), a radiopharmaceutical diagnostic agent used in PET imaging to visualize PSMA-positive lesions in men with prostate cancer with suspected metastasis or recurrence.
💊 Sotagliflozin (Inpefa), a dual SGLT1/2 inhibitor used to reduce the risk of CV, hospitalization for heart failure, and urgent heart failure visit in adults with heart failure or type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors
💉Glofitamab (Columvi), a bispecific mAb directed against CD20 and CD3 which is used for the treatment of relapsed or refractory diffuse large B-cell lymphoma.
💊 Ritlecitinib (Litfulo), a dual JAK3/TEC kinase family inhibitor used to treat severe alopecia areata in adults and adolescents 12 years and older.
💉Rozanolixizumab (Rystiggo), a humanized mAb targeting the human neonatal Fc receptor (FcRn) used to treat generalized myasthenia gravis.
💉Somatrogon (Ngenla), a long-acting recombinant human growth hormone used as the long-term treatment of pediatric patients who have growth failure due to growth hormone deficiency.
Again, a fascinating collection from Chris de Savi!