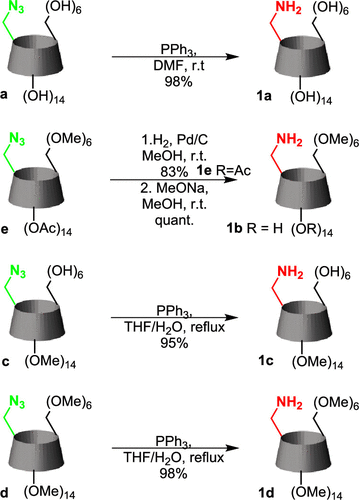
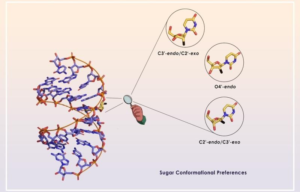
Ribofuranose sugar conformation plays an important role in the structure and dynamics of functional nucleic acids such as siRNAs, AONs, aptamers, miRNAs, etc. Several chemical modifications have been introduced into sugar moiety to improve their therapeutic potential over the years. The stability of the oligonucleotide duplexes as well as the formation of stable and functional protein-oligonucleotide complexes are dictated by the conformation and dynamics of the sugar moiety. In this review, Gourav Das Harikrishna and Kiran Gore systematically categorize various ribofuranose sugar modifications employed in DNAs and RNAs so far. We discuss different stereoelectronic effects imparted by different substituents on the sugar ring and how these effects control sugar puckering. Using this data, it would be possible to predict the precise use of chemical modifications and design novel sugar-modified nucleosides for therapeutic oligonucleotides that can improve their physicochemical properties.
See the full article here: Influence of Sugar Modifications on the Nucleoside Conformation and Oligonucleotide Stability: A Critical Review