CarboHyde at CPHI Barcelona 2023
What a great event CPHI has been this year again! Nothing to compare with the vibe of 40,000 visitors, 1800 exhibitors, 50+meetings in 3 days and over 40 km walked.
I hope we will meet in Milano at 2024!










What a great event CPHI has been this year again! Nothing to compare with the vibe of 40,000 visitors, 1800 exhibitors, 50+meetings in 3 days and over 40 km walked.
I hope we will meet in Milano at 2024!










Today’s cyclodextrin is a great review on tastemasking potential by Lena Adamkiewicz and Łukasz Szeleszczuk.
A systematic evaluation was conducted, which resulted in the selection of 67 works representing both successful and unsuccessful works describing the application of CDs as taste-masking excipients. Particular attention has been given to the methods of evaluation of the taste-masking properties and the factors affecting the outcomes, such as the choice of the proper cyclodextrin or guest–host molar ratio. The conclusions of this review reveal that the application of CDs is not straightforward; nevertheless, this solution can be an effective, safe, and inexpensive method of taste masking for pharmaceutical purposes.

Paul Fehlner, co-founder and CEO of reVision Therapeutics, Inc., provided an overview of our their program, REV-0100, for Stargardt disease, a rare pediatric retinal disease. REV-0100 has the potential to expand to dry age-related macular degeneration (AMD). During the session, he discussed various aspects of REV-0100, including its mechanism of action, in vivo efficacy in Stargardt mouse models, systemic safety, competitive advantage, and development timeline.
REV-0100 is sulfobutyl-ether-beta-cyclodextrin, a commonly used pharmaceutical excipient now evaluated in this orphan indication.
We invite you to join our webinar event, which explores practical areas of developing cyclodextrin-based formulations such as manufacturing, regulatory and safety aspects along with novel applications like using them as active ingredients.
This series of educative sessions offers a comprehensive examination of the current state and future prospects of utilizing cyclodextrins in various domains, with a special focus on their use in formulations and as therapeutic agents.
Please help our work to fill this form, thanks in advance!
https://forms.gle/te1BBTgrZQ3cYgUT7
You can check it on YouTube:
 Carmen Popescu – Global Pharma/Bio Technical Developer at Roquette
Carmen Popescu – Global Pharma/Bio Technical Developer at Roquette
 Julien Parcq – Manager of Functionalization of Starches, Sugars and Polyols Department at Roquette
Julien Parcq – Manager of Functionalization of Starches, Sugars and Polyols Department at Roquette
 Damien Truffin – Toxicologist Expert at Roquette
Damien Truffin – Toxicologist Expert at Roquette
 Tamas Sohajada – CEO at CarboHyde
Tamas Sohajada – CEO at CarboHyde
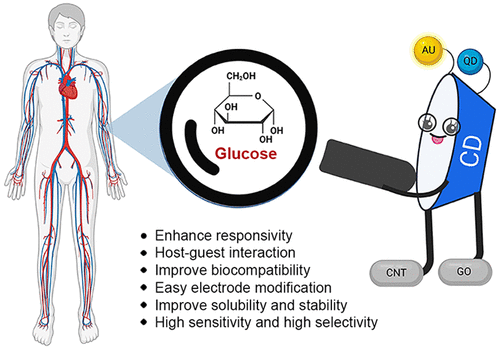
today’s cyclodextrin: is about glucose sensors from Abolfazl Heydari et al.
Monitoring blood glucose levels is crucial in diabetes management, aiding in clinical decision making and reducing the incidence of hypoglycemic episodes, thereby decreasing morbidity and mortality rates. Despite advancements in glucose monitoring (GM), the development of noninvasive, rapid, accurate, sensitive, selective, and stable systems for continuous monitoring remains a challenge.
In this concept, cyclodextrins (CDs) can be instrumental in the development of GM systems due to their high supramolecular recognition capabilities based on the host–guest interaction. The introduction of CDs into GM systems not only impacts the sensitivity, selectivity, and detection limit of the monitoring process but also improves biocompatibility and stability. We can categorize CD-based sensors into four groups based on their modification strategies, including CD-modified boronic acid, CD-modified mediators, CD-modified nanoparticles, and CD-modified functionalized polymers. These findings shed light on the potential of CD-based sensors as a promising tool for continuous GM in diabetes mellitus management.
Cyclodextrin Host–Guest Recognition in Glucose-Monitoring Sensors | ACS Omega
Exploring alpha-Cyclodextrin for weight loss and metabolic benefit in mice with associated increases in GLP-1 secretion obesity diabetes- Fascinating study by Københavns Universitet – University of Copenhagen.
Based on all that has been shared on ACD and its effect on weight management and diabetes including clinical data, do you think this could turn to a drug eventually?

today’s cyclodextrin is a cool review from Universidad Complutense de Madrid on the formation of inclusion complexes with drugs belonging to class IV, the synthesis of chemically modified cyclodextrins, polymers, and nanosponges and analytical techniques that allow the characterization and verification of the formation of true inclusion complexes.

Fascinating invention from Venn Biosciences using machine learning to predict glycopeptide fragmentation patterns and retention times. Do you also use AI in your pharma development?
De novo glycopeptide sequencing

Breaking news!
Alveron Pharma announced that it has completed a Phase 1 clinical study for OKL-1111, a new drug for the rapid treatment of Intracranial Haemorrhage (ICH) and other life-threatening bleeds associated with the use of anticoagulants or platelet inhibitors. OKL-1111 was well-tolerated in the trial with healthy human volunteers and showed no more adverse events above those in the placebo groups. Volunteers also received an anticoagulant and a pharmacodynamic effect was observed with OKL-1111 administration. In the prior non-clinical program, the drug reduced bleeding in a clinically relevant intracranial haemorrhage model using high doses of an anticoagulant. Furthermore, a broad-spectrum mode of action was demonstrated against all classes of anticoagulant and one platelet inhibitor to date in a standard haemostasis model.
Congratulations! Way to go!
Alveron Pharma completes successful first-in-human trial of OKL-1111

Today’s cyclodextrin:
The great potential of the Aquilon Pharma‘s innovative inhaled solution of budesonide-cyclodextrin is clearly demonstrated on this poster from Didier Cataldo on the main outcomes of the Boreas study on both efficacy and safety.
