Finally, a novel cyclodextrin derivative in human clinics again!
Breaking news!
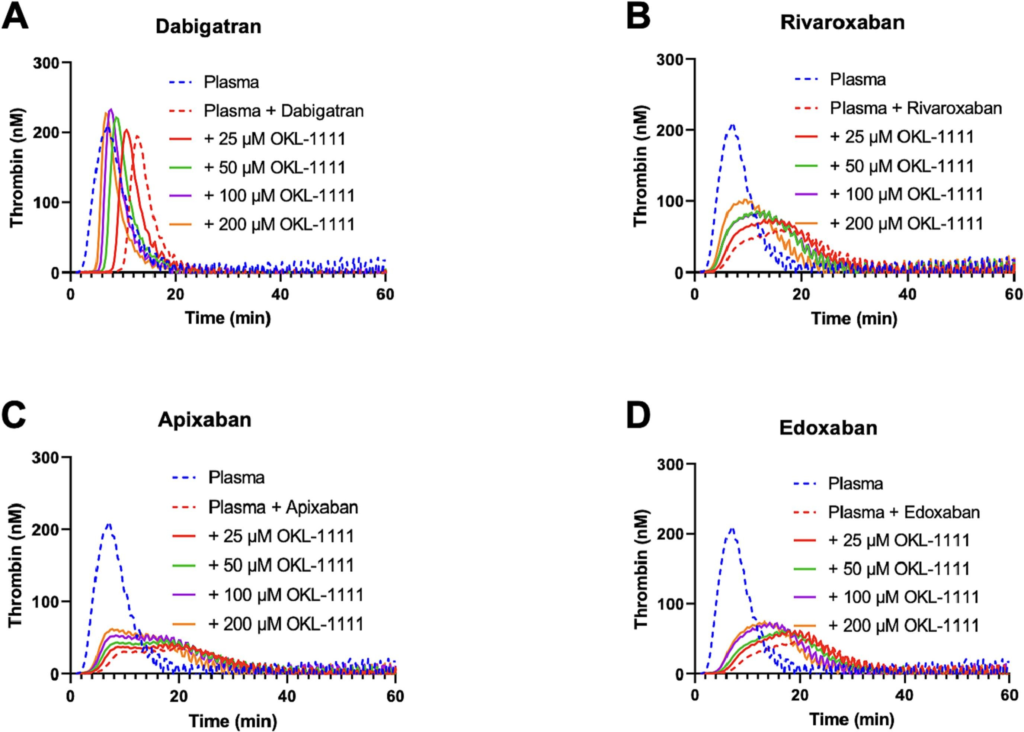
Alveron Pharma announced that it has completed a Phase 1 clinical study for OKL-1111, a new drug for the rapid treatment of Intracranial Haemorrhage (ICH) and other life-threatening bleeds associated with the use of anticoagulants or platelet inhibitors. OKL-1111 was well-tolerated in the trial with healthy human volunteers and showed no more adverse events above those in the placebo groups. Volunteers also received an anticoagulant and a pharmacodynamic effect was observed with OKL-1111 administration. In the prior non-clinical program, the drug reduced bleeding in a clinically relevant intracranial haemorrhage model using high doses of an anticoagulant. Furthermore, a broad-spectrum mode of action was demonstrated against all classes of anticoagulant and one platelet inhibitor to date in a standard haemostasis model.
Congratulations! Way to go!
Alveron Pharma completes successful first-in-human trial of OKL-1111