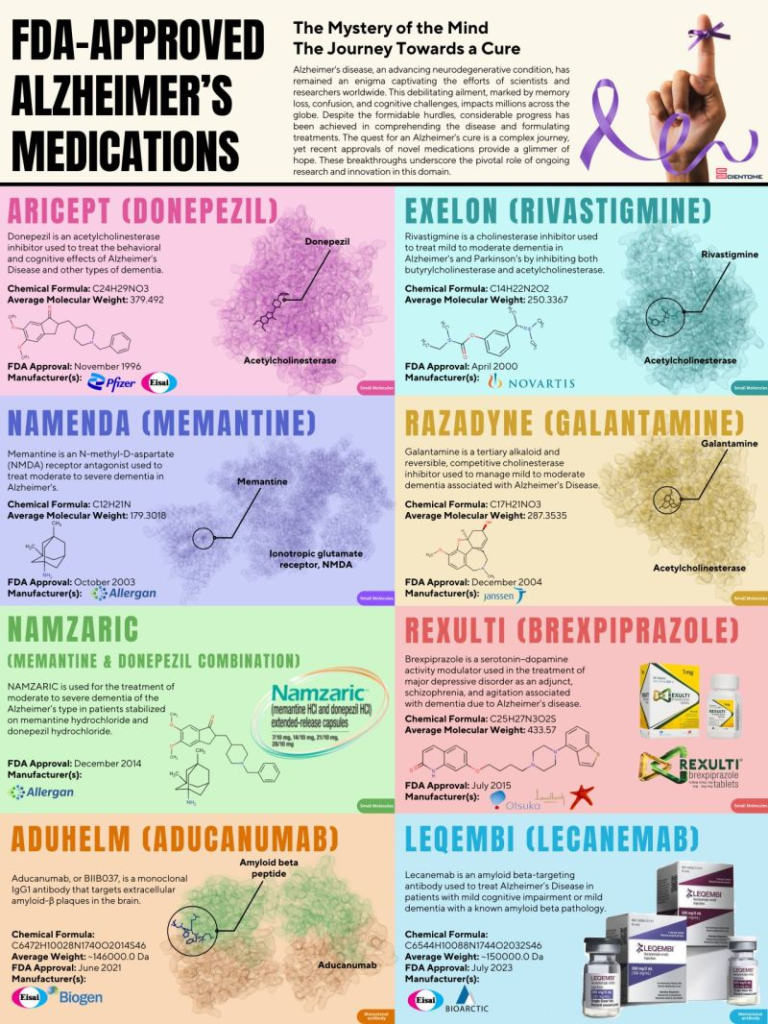
FDA Approves Lilly’s Kisunla™ (donanemab-azbt) for Early Symptomatic Alzheimer’s Disease Treatment
As a company also involved in research for Alzheimer’s disease both from the DDS and API perspective, we cheer for this news!
The U.S. Food and Drug Administration (FDA) has approved Kisunla™ (donanemab-azbt), a once-monthly injection (350 mg/20 mL) for IV infusion developed by Eli Lilly and Company.
Kisunla is designed to treat adults with early symptomatic Alzheimer’s disease (AD), which includes mild cognitive impairment (MCI) and mild dementia stages of AD, with confirmed amyloid pathology. This is the first and only amyloid plaque-targeting therapy that allows stopping treatment when amyloid plaques are cleared, potentially reducing costs and the number of infusions needed.
FDA approves new drug to treat Alzheimer’s disease (nbcnews.com)