Novel targets for immune-checkpoint inhibition in cancer
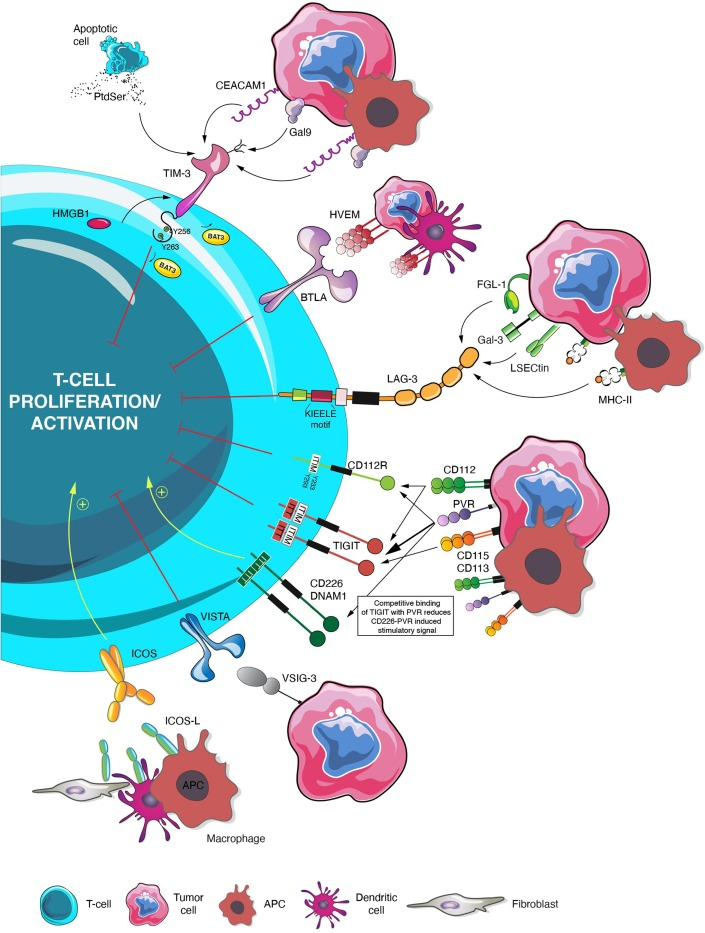
Immune-checkpoint inhibitors have revolutionized cancer therapy, yet many patients either do not derive any benefit from treatment or develop a resistance to checkpoint inhibitors. Intrinsic resistance can result from neoantigen depletion, defective antigen presentation, PD-L1 downregulation, immune-checkpoint ligand upregulation, immunosuppression, and tumor cell phenotypic changes. On the other hand, extrinsic resistance involves acquired upregulation of inhibitory immune-checkpoints, leading to T-cell exhaustion. Current data suggest that PD-1, CTLA-4, and LAG-3 upregulation limits the efficacy of single-agent immune-checkpoint inhibitors. Ongoing clinical trials are investigating novel immune-checkpoint targets to avoid or overcome resistance. This review provides an in-depth analysis of the evolving landscape of potentially targetable immune-checkpoints in cancer, highlight their biology, emphasizes the current understanding of resistance mechanisms, and focuses on promising strategies that are under investigation. Current results and ongoing clinical trials in this crucial field that could once again revolutionize outcomes for cancer patients are also collected.
Novel targets for immune-checkpoint inhibition in cancer – Cancer Treatment Reviews