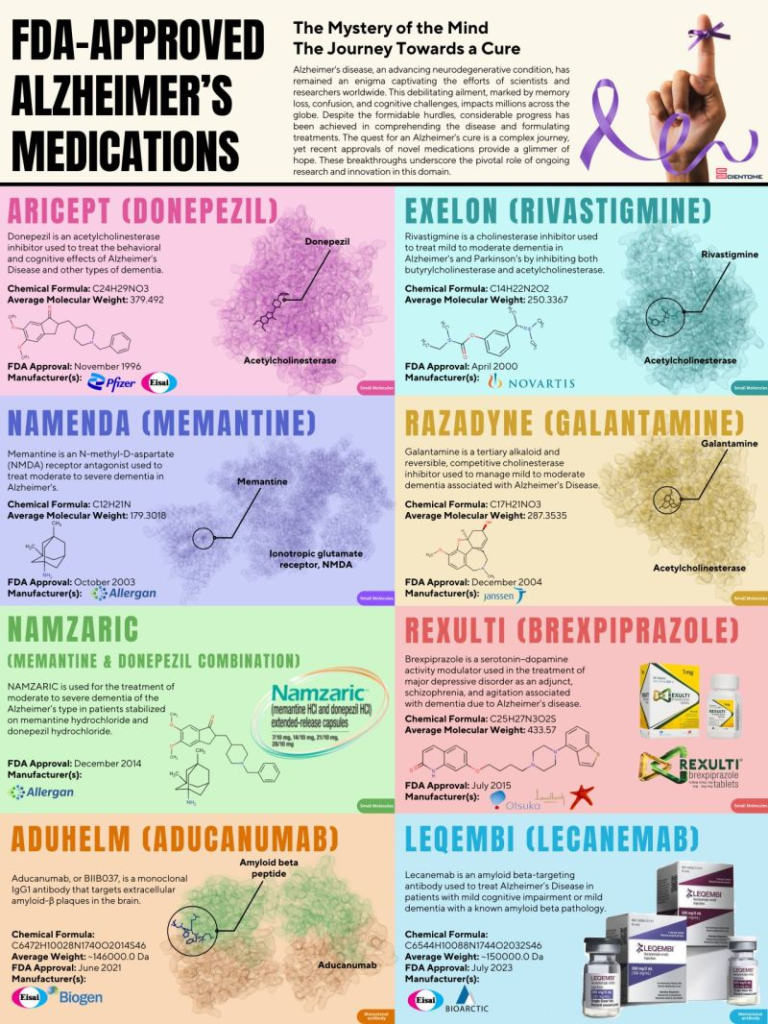
FDA-Approved Alzheimer’s Medications
⚫ Alzheimer’s disease takes a toll on not just the people suffering from the disease but also on their loved ones and caregivers in a way that almost no other illness does.
⚫ The Alzheimer’s disease market is projected to experience robust growth at a compound annual growth rate of 20% from $2.2 billion in 2020 to $13.7 billion in 2030!
🔶 Drug Development Pipeline:
🔸 187 current trials assessing 141 unique drugs
🔸 Phase 3: 36 drugs
🔸 Phase 2: 87 drugs
🔸 Phase 1: 31 drugs
🔷 Phase 3 has 36 agents in 55 trials:
🔹 DMT*-Biologics: 9 (25%)
🔹 DMT*-Small molecules: 15 (42%)
🔹 Putative cognitive-enhancers: 5 (14%)
🔹 Neuropsychiatric symptoms: 7 (19%)
*DMT stands for disease-modifying therapies
🔶 Most Common Targets:
🔸 Transmitter receptors, amyloid, synaptic function, and inflammation
🔷 58 new drugs have entered the pipeline in the past year!
🔷 28% of candidate therapies are repurposed from other diseases!
⚫Despite the hype, controversy, and occasional scandal surrounding the approval of Alzheimer’s drugs, and the disappointing reports of the ineffectiveness of recently approved drugs, many companies are still dedicating time and resources to finding a potential cure for this debilitating disease. This gives hope that the future is bright for those living with Alzheimer’s.
🔶 Learn more:
🔸 Alzheimer’s disease drug development pipeline: 2023 – Cummings – 2023 – Alzheimer’s & Dementia: Translational Research & Clinical Interventions – Wiley Online Library
🔸 Alzheimer’s Drug Market To Hit $13B As FDA Approvals And Insurance Coverage Escalate (forbes.com)
Great collection from Maryam Daneshpour