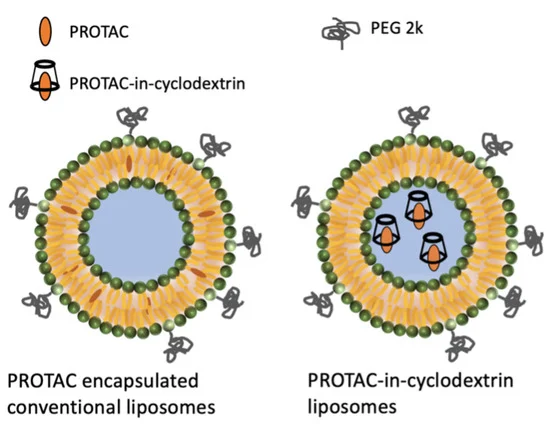
Proteolysis-Targeting Chimeras (PROTACs) are a promising new technology in drug development. They have rapidly evolved in recent years, with several of them in clinical trials. While most of these advances have been associated with monovalent protein degraders, bivalent PROTACs have also entered clinical trials, although progression to market has been limited. One of the reasons is the complex physicochemical properties of the heterobifunctional PROTACs. A promising strategy to improve pharmacokinetics of highly lipophilic compounds, such as PROTACs, is encapsulation in liposome systems. Here liposome systems for intravenous administration to enhance the PK properties of two bivalent PROTAC molecules are described, by reducing clearance and increasing systemic coverage. A PROTAC-in-cyclodextrin liposome system was developed where the drug was retained in the liposome core. In PK studies at 1 mg/kg for GNE-01 the PROTAC-in-cyclodextrin liposome, compared to the solution formulation, showed a 80- and a 380-fold enhancement in AUC for mouse and rat studies, respectively. We further investigated the same PROTAC-in-cyclodextrin liposome system with the second PROTAC (GNE-02), where we monitored both lipid and drug concentrations in vivo. Similarly, in a mouse PK study of GEN-02, the PROTAC-in-cyclodextrin liposome system exhibited enhancement in plasma concentration of a 23× increase over the conventional solution formulation. Importantly, the lipid CL correlated with the drug CL. Additionally, we investigated a conventional liposome approach for GNE-02, where the PROTAC resides in the lipid bilayer. Here, a 5× increase in AUC was observed, compared to the conventional solution formulation, and the drug CL was faster than the lipid CL. These results indicate that the different liposome systems can be tailored to translate across multiple PROTAC systems to modulate and improve plasma concentrations. Optimization of the liposomes could further improve tumor concentration and improve the overall therapeutic index (TI). This delivery technology may be well suited to bring novel protein targeted PROTACs into clinics.
This is a unique industrial collaboration between Genentech, Arvinas & Bristol Myers Squibb.
#cyclodextrin #liposome #drugdelivery
Pharmaceutics | Free Full-Text | Development of Liposome Systems for Enhancing the PK Properties of Bivalent PROTACs (mdpi.com)