The electrochemical quantitation method for sugammadex via a molecularly imprinted polymer-based sensor
Sugammadex (SUG) is a synthetically modified γ-cyclodextrin derivative used in hospitals after surgeries to reverse the neuromuscular blockade induced by rocuronium or vecuronium. Who has experience in the field knows that analysis (in particular those human samples) of carbohydrates and cyclodextrin are very challenging.
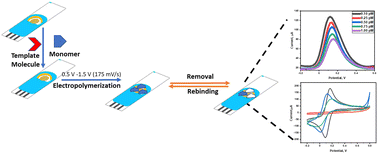
In this study, Ankara University and DEVA Holding A.Ş. present the first electroanalytical quantification method for sugammadex by using molecular imprinting (MIP) via the electropolymerization (EP) technique. An EP-MIP film was formed by EP on a screen-printed gold electrode (SPAuE) and a new electrochemical sensor, EP-MIP(SUG)/SPAuE, was fabricated using the 4-aminophenol monomer with copper ions to enhance the MIP-binding site. Surface and electrochemical characterization of the EP-MIP(SUG)/SPAuE sensor have been done via scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The specificity/selectivity of the developed sensor has been shown by using common interferents found in the biological fluids and also molecules having similar structures, such as α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin. As a result, a quantitative analysis method has been developed and validated by using the EP-MIP(SUG)/SPAuE sensor in the concentration range of 0.1–1.0 pM with very high sensitivity (limit of detection: 27.3 fM). The applicability of the method has been shown for bulk drug substances, pharmaceutical dosage forms, and commercial serum samples.
Göksu Özçelikay, AHMET CETINKAYA, Esen BELLUR ATİCİ, Sibel A. OZKAN