Single Residue Variation in Skeletal Muscle Myosin Enables Direct and Selective Drug Targeting for Spasticity and Muscle Stiffness
Hungary is just a small grain on the beach of the pharma industry, yet from time to time we also make remarkable discoveries.
Today, its Motorpharma Ltd. a spin-off from Eötvös Loránd University that makes us proud by starting to dose their lead compounds MPH-220 to humans in Texas.
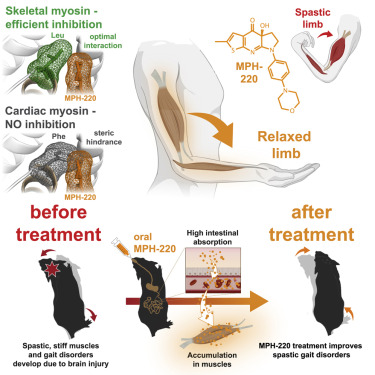
Mate Gyimesi, András Málnási-Csizmadia et al first published in 2020 in Cell about MPH-220, whish is targeting skeletal muscle myosin and can enabled muscle relaxation, in human and model systems, without cardiovascular side effects and improved spastic gait disorders after brain injury in a disease model. MPH-220 provides a potential nervous-system-independent option to treat spasticity and muscle stiffness.
Muscle spasticity after nervous system injuries and painful low back spasm affect more than 10% of global population. Current medications are of limited efficacy and cause neurological and cardiovascular side effects because they target upstream regulators of muscle contraction. Direct myosin inhibition could provide optimal muscle relaxation; however, targeting skeletal myosin is particularly challenging because of its similarity to the cardiac isoform.
About Motorpharma: https://motorpharma.com/