Effect of Substitution Degree and Homogeneity on Cyclodextrin-Ligand Complex Stability: Comparison of Fenbufen and Fenoprofen Using CD and NMR Spectroscopy
today’s cyclodextrin:
is a rarely applied analytical technique for the analysis of CD-complexes: circular dichroism, presented by the group at Semmelweis University where I got my PhD, so close to my heart.
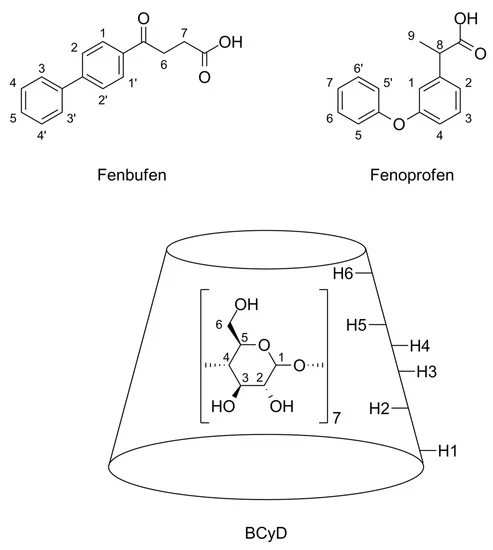
The stability of host–guest complexes of two NSAID drugs with similar physicochemical properties, fenbufen and fenoprofen, was investigated by comparing induced circular dichroism and 1H nuclear magnetic resonance methods using eight cyclodextrins of different degrees of substitution and isomeric purity as guest compounds. These cyclodextrins include native β-cyclodextrin (BCyD), 2,6-dimethyl-β-cyclodextrin 50 (DIMEB50), 80 (DIMEB80) and 95% (DIMEB95) isomerically pure versions, low-methylated CRYSMEB, randomly methylated β-cyclodextrin (RAMEB) and 4.5 and 6.3 average substitution grade hydroxypropyl-β-cyclodextrin (HPBCyD). The stability constants obtained by the two methods show good agreement in most cases. For fenbufen complexes, there is a clear trend that the stability constant increases with the degree of substitution, while isomer purity has a smaller effect on the magnitude of stability constants.
Mazákné Dr. Kraszni Márta, Ferenc Agh, Daniel Horvath, Arash Mirzahosseini, Horváth Péter
See the full article here: Effect of Substitution Degree and Homogeneity on Cyclodextrin-Ligand Complex Stability: Comparison of Fenbufen and Fenoprofen Using CD and NMR Spectroscopy