This is a fascinating question:
Are LNPs of mRNA drugs mere excipients or part of the drug?
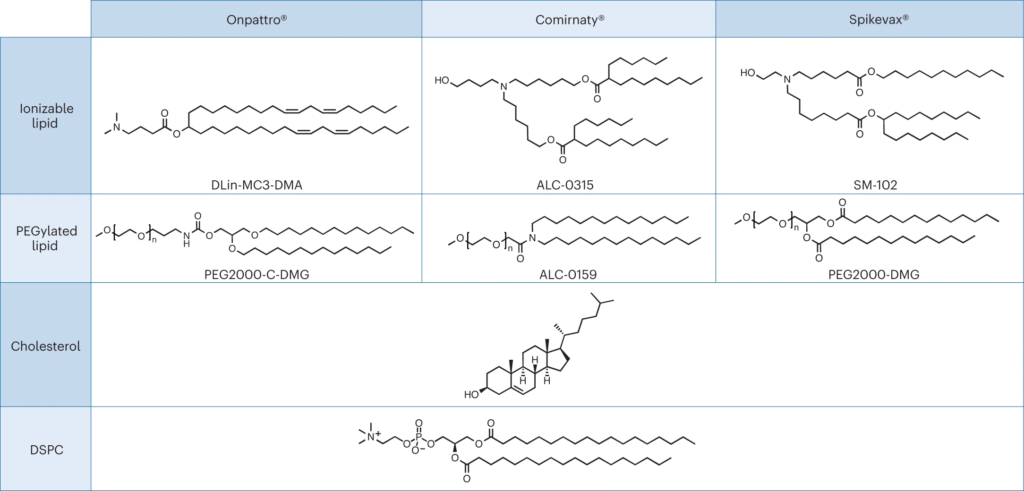
Nanomedicines are complex drugs where components that have typically been regarded as excipients may now be considered part of the active ingredient. The distinction between the active ingredient and excipients for nanomedicines has important consequences for regulatory review and product development. The dissimilarity in the review of the recent RNA-based lipid nanoparticles highlights the need for further regulatory alignment on this topic.
To aid with this journey, both the US FDA and the European Medicines Agency have produced helpful guidelines. These documents, some specifically related to nanomedicines, provide the drug developer with a framework for selecting the appropriate regulatory path towards clinical trials and eventual product approval. The relevant pathway will then determine the type and amount of data that will be required for regulatory review. For example, a novel drug will likely require full Phase I to Phase III clinical trials, whereas a nano-formulation of an existing drug may qualify for an abridged/abbreviated review or as a generic formulation.
Dr. Eva Hemmrich & Scott McNeil