today’s cyclodextrin:
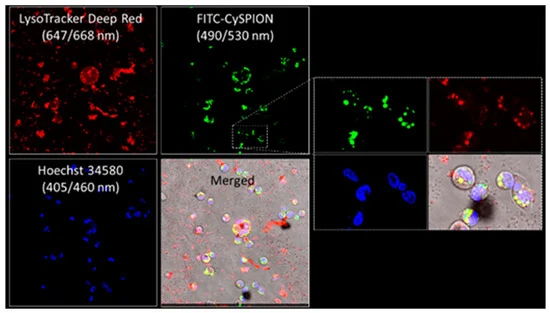
Core–shell superparamagnetic iron oxide nanoparticles hold great promise as a theranostic platform in biological systems. Antonino Puglisi and coworkers report the biological effect of multifunctional cyclodextrin-appended SPIONs (CySPION) in mutant Npc1-deficient CHO cells compared to their wild-type counterparts. CySPIONs show negligible cytotoxicity while they are strongly endocytosed and localized in the lysosomal compartment. Through their bespoke pH-sensitive chemistry, these nanoparticles release appended monomeric cyclodextrins to mobilize over-accumulated cholesterol and eject it outside the cells. CySPIONs show a high rate of transport across blood–brain barrier models, indicating their promise as a therapeutic approach for cholesterol-impaired diseases affecting the brain.
University of Natural Resources and Life Sciences, Vienna (BOKU): Peter van Oostrum, Erik Reimhult
Università degli Studi di Catania: Noemi Bognanni, Graziella Vecchio
Ege University: Ece Bayir
University of Oxford: Dawn Shepherd, Frances Platt
See the full article here: Grafting of Cyclodextrin to Theranostic Nanoparticles Improves Blood-Brain Barrier Model Crossing